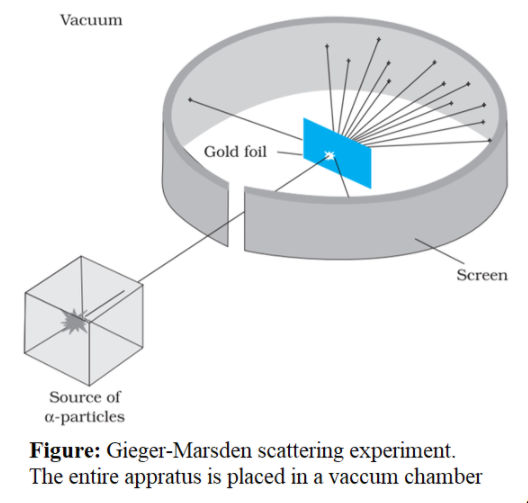
What Is The Meaning Of Gold Foil Experiment . rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. ernest rutherford's famous gold foil experiment involves the. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking.
from www.animalia-life.club
Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. ernest rutherford's famous gold foil experiment involves the.
Rutherford Gold Foil Experiment
What Is The Meaning Of Gold Foil Experiment the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a piece of gold foil was hit with alpha particles, which have a positive charge. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. ernest rutherford's famous gold foil experiment involves the. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Most alpha particles went right through. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking.
From chemistrysubject.blogspot.com
Chemistry Subject Rutherford's Gold Foil Experiment What Is The Meaning Of Gold Foil Experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Most alpha particles went right through. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a piece of gold foil was hit with. What Is The Meaning Of Gold Foil Experiment.
From pediaa.com
What is Rutherford's Gold Foil Experiment What Is The Meaning Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. ernest rutherford's famous gold foil experiment involves the. Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. to verify his. What Is The Meaning Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment What Is The Meaning Of Gold Foil Experiment Most alpha particles went right through. ernest rutherford's famous gold foil experiment involves the. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. the gold foil. What Is The Meaning Of Gold Foil Experiment.
From study.com
Ernest Rutherford's Gold Foil Experiment Physics Lab Video & Lesson What Is The Meaning Of Gold Foil Experiment to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment in 1911,. What Is The Meaning Of Gold Foil Experiment.
From ernestrutherfordsite.weebly.com
The Gold Foil Experiment Ernest Rutherford What Is The Meaning Of Gold Foil Experiment to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a piece of gold foil was hit with alpha particles, which have a positive charge. ernest. What Is The Meaning Of Gold Foil Experiment.
From large.stanford.edu
Experimental Evidence for the Structure of the Atom What Is The Meaning Of Gold Foil Experiment ernest rutherford's famous gold foil experiment involves the. a piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. rutherford's gold foil experiment led to the discovery. What Is The Meaning Of Gold Foil Experiment.
From historyoftheatom.wordpress.com
Ernest Rutherford (1911) HISTORY OF THE ATOM What Is The Meaning Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. ernest rutherford's famous gold foil experiment involves the. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. the gold foil experiment in 1911, rutherford and coworkers hans geiger and. What Is The Meaning Of Gold Foil Experiment.
From www.shutterstock.com
Rutherford Gold Foil Experiment Infographic Diagram 库存矢量图(免版税 What Is The Meaning Of Gold Foil Experiment ernest rutherford's famous gold foil experiment involves the. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. the gold foil. What Is The Meaning Of Gold Foil Experiment.
From www.youtube.com
Rutherford Gold Foil Experiment My Inter Academy YouTube What Is The Meaning Of Gold Foil Experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. ernest rutherford's famous gold foil experiment involves the. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. the gold foil. What Is The Meaning Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment What Is The Meaning Of Gold Foil Experiment the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in. What Is The Meaning Of Gold Foil Experiment.
From www.youtube.com
Gold Foil Technique YouTube What Is The Meaning Of Gold Foil Experiment ernest rutherford's famous gold foil experiment involves the. Most alpha particles went right through. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus.. What Is The Meaning Of Gold Foil Experiment.
From www.youtube.com
Rutherford's Gold Foil Experiment Quick and Simple! YouTube What Is The Meaning Of Gold Foil Experiment the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in. What Is The Meaning Of Gold Foil Experiment.
From www.animalia-life.club
Rutherford Gold Foil Experiment What Is The Meaning Of Gold Foil Experiment ernest rutherford's famous gold foil experiment involves the. Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. rutherford's gold foil experiment led to the discovery. What Is The Meaning Of Gold Foil Experiment.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii What Is The Meaning Of Gold Foil Experiment a piece of gold foil was hit with alpha particles, which have a positive charge. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of. What Is The Meaning Of Gold Foil Experiment.
From chemistryhungergames.com
Chemistry Hunger Games District 1 What Is The Meaning Of Gold Foil Experiment Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. ernest rutherford's famous gold foil experiment involves the.. What Is The Meaning Of Gold Foil Experiment.
From wisc.pb.unizin.org
Rutherford’s Experiments (M2Q2) UWMadison Chemistry 103/104 Resource What Is The Meaning Of Gold Foil Experiment the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a piece of gold foil was hit with alpha particles, which have a positive charge. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the. What Is The Meaning Of Gold Foil Experiment.
From www.visionlearning.com
Atomic Theory I Chemistry Visionlearning What Is The Meaning Of Gold Foil Experiment Most alpha particles went right through. the gold foil experiment in 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. ernest rutherford's famous gold foil experiment involves the.. What Is The Meaning Of Gold Foil Experiment.
From ar.inspiredpencil.com
Gold Foil Experiment Animation What Is The Meaning Of Gold Foil Experiment Most alpha particles went right through. to verify his model, rutherford developed a scientific model to predict the intensity of alpha particles at the different angles they. ernest rutherford's famous gold foil experiment involves the. a piece of gold foil was hit with alpha particles, which have a positive charge. the gold foil experiment in 1911,. What Is The Meaning Of Gold Foil Experiment.